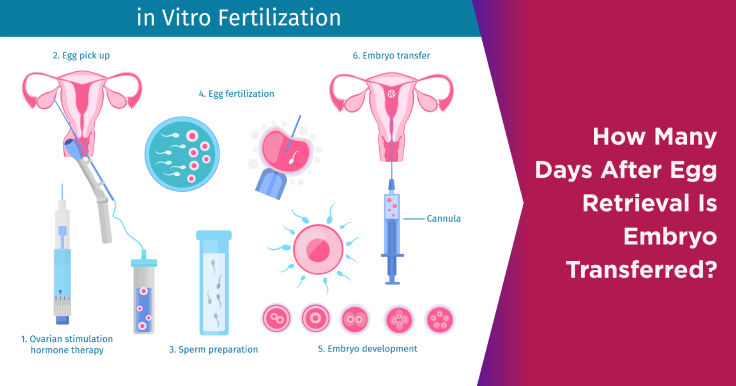
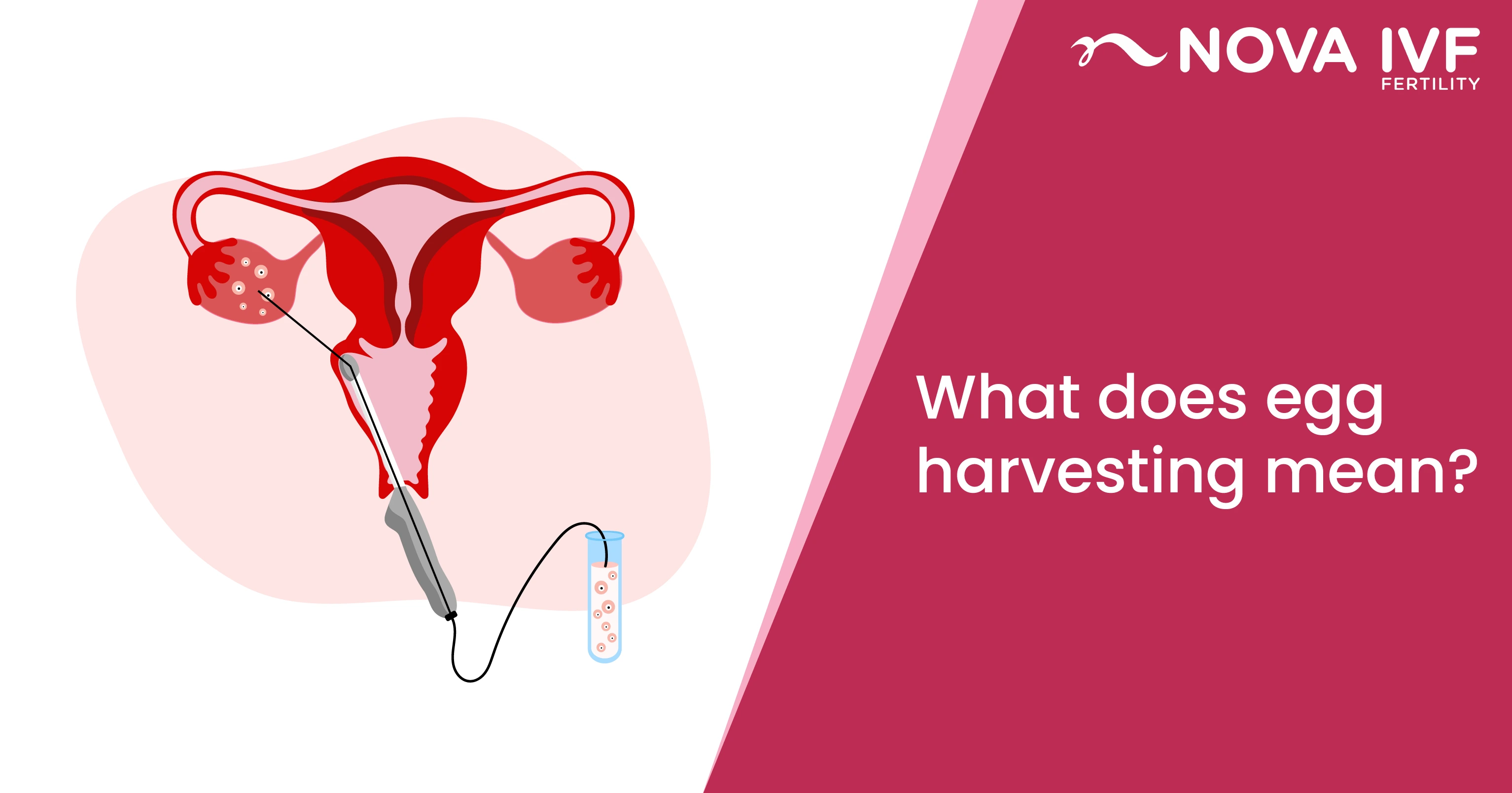
Embryology Lab Management: Best Practices for Success

Couples opting to have children through IVF place a lot of faith in fertility clinics and their embryology labs. Thus, it is essential for these labs to be run professionally and managed well. While there are no laws governing how IVF labs should be run there are certain ICMR guidelines for IVF Lab management including the below-listed ones:
Distinct Sterile and Non-sterile Areas
IVF labs should have separate sterile and non-sterile areas with no overlap between them. The sterile areas include the lab area where the embryo is developed and incubated, semen processing lab and IUI cleanroom. These areas should be air-conditioned with 100% power backup. Non-sterile areas include the reception, waiting room, general lab etc. Entry to the sterile areas of the lab should be restricted to embryologists and doctors in sterile garments and footwear.
Staffing
The number of doctors, nurses, embryologists etc. should reflect the number of IVF procedures performed each year. A clinic that performs up to 150 IVF cycles annually should have at least 2 full-time embryologists on the panel. This enables doctors to retrieve eggs and sperm when they are healthiest and not based on the availability of the embryologist.
Quality Management
All equipment used should be serviced periodically. Any media, reagents or disposables being used should be tested from time to time for any pH change Similarly, all cells, tissues and material used should be recorded for full transparency. Every procedure performed in the lab should be double witnessed. This reduces the risk of mix-ups and human error. The embryologist is usually the person responsible for checking quality management.
Records
A good IVF lab should maintain up-to-date records of all procedures performed. This includes procedure dates, how each procedure was performed, staff involved, characteristics of embryos and any relevant communication with the patient. Any handwritten or electronic edits made to these records should be traceable.
 Infertility Counselling
Infertility Counselling Female Infertility Treatment
Female Infertility Treatment Andrology Treatment
Andrology Treatment Fertility Enhancing Surgeries - Female
Fertility Enhancing Surgeries - Female Fertility Enhancing Surgeries - Male
Fertility Enhancing Surgeries - Male Endoscopy Treatment
Endoscopy Treatment IUI Treatment
IUI Treatment IVF Treatment
IVF Treatment ICSI Treatment
ICSI Treatment Advanced IVF Solutions
Advanced IVF Solutions Embryology
Embryology Vitrification Egg, Embryo, Sperm Freezing
Vitrification Egg, Embryo, Sperm Freezing Preimplantation Genetic Testing (PGT)
Preimplantation Genetic Testing (PGT) Donation Program Embryo / Egg / Sperm
Donation Program Embryo / Egg / Sperm Self-cycleTM IVF
Self-cycleTM IVF

 Self-cycleTM IVF
Self-cycleTM IVF